Introduction
Limit tests in pharmaceutical analysis are essential quality control procedures used to detect and quantify trace impurities in drugs and raw materials. These tests ensure compliance with pharmacopeial standards, guaranteeing drug safety and efficacy. The United States Pharmacopeia (USP), British Pharmacopoeia (BP), and Indian Pharmacopoeia (IP) set stringent limits for impurities such as heavy metals, arsenic, Sulfates, and chlorides.
This article explores the principles, methods, and significance of limit tests in pharmaceutical analysis.
What Are Limit Tests in Pharmaceutical Analysis?
Limit tests are semi-quantitative or qualitative analytical procedures designed to identify impurities in pharmaceutical substances at levels that do not affect drug safety or efficacy. Unlike assays, which measure active ingredients, limit tests detect contaminants below a predefined threshold.

Importance of Limit Tests in Pharmaceuticals
- Regulatory Compliance: Ensures adherence to pharmacopeial standards (USP, BP, IP).
- Drug Safety: Prevents toxic effects due to contaminants like heavy metals and arsenic.
- Product Quality Assurance: Maintains consistency in drug formulations.
- Environmental Safety: Reduces pharmaceutical pollution by monitoring contaminants.
Common Limit Tests in Pharmaceutical Analysis
Pharmacopoeias prescribe specific tests for different impurities. Below are some of the most important limit tests:
1. Limit Test for Heavy Metals
Principle: Detects traces of heavy metals (lead, mercury, cadmium) using sulfide precipitation or color comparison methods.
Procedure:
- The sample is dissolved in an acidic medium.
- A reagent like thioacetamide or hydrogen sulfide (H₂S) reacts with metal ions, forming a black precipitate.
- The intensity of the color is compared against a standard lead solution.
Significance: Prevents toxic metal accumulation, which can cause neurological damage and organ failure.
2. Limit Test for Arsenic
Principle: Based on the Gutzeit test, arsenic reacts with hydrogen to form arsine gas, producing a yellow to brown color on mercuric chloride paper.
Procedure:
- The sample is mixed with stannous chloride and zinc in an acidic medium.
- Arsenic forms arsine gas (AsH₃), which reacts with HgCl₂ paper, turning it yellow/brown.
- The result is compared to a standard.
- Significance: Ensures compliance with WHO arsenic limits, preventing carcinogenic risks.
3. Limit Test for Sulfates
Principle: Detects excess sulfate using barium chloride (BaCl₂) to form a white precipitate of barium sulfate (BaSO₄).
Procedure:
- The sample solution is mixed with barium chloride in acidic conditions.
- The formation of a white precipitate indicates the presence of Sulfates.
- The intensity is compared with a standard sulfate solution.
- Significance: Controls sulfate contamination, which affects drug solubility and stability.
4. Limit Test for Chlorides
Principle: Based on precipitation of silver chloride (AgCl) using silver nitrate (AgNO₃).
Procedure:
- The sample is dissolved in nitric acid.
- Silver nitrate solution is added, forming a white precipitate.
- The result is compared with a standard chloride solution.
- Significance: Prevents chloride-related degradation and instability in pharmaceuticals.
5. Limit Test for Iron
Principle: Uses thioglycolic acid or ammonium thiocyanate, forming a red-colored ferric thiocyanate complex.
Procedure:
- The sample is treated with acid and thioglycolic acid.
- A red color develops, indicating iron contamination.
- The intensity is compared with a standard.
- Significance: Ensures that excess iron does not affect drug stability or efficacy.
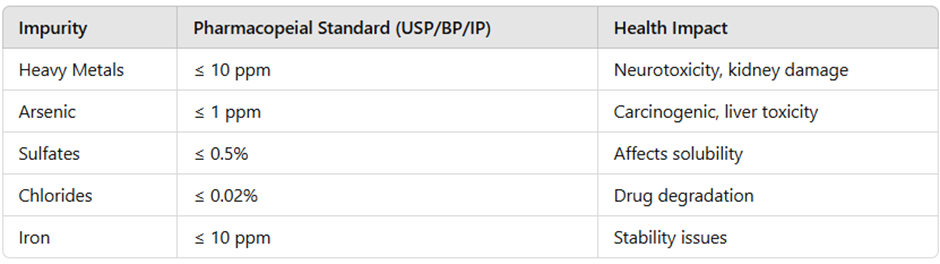
Pharmacopeial Standards for Limit Tests
Advancements in Limit Testing
Modern analytical techniques improve the accuracy and sensitivity of limit tests:
- Atomic Absorption Spectroscopy (AAS): Detects trace metals with high precision.
- Inductively Coupled Plasma Mass Spectrometry (ICP-MS): Provides ultra-sensitive impurity detection.
- UV-Vis Spectrophotometry: Enhances colorimetric limit test accuracy.
Conclusion
Limit tests in pharmaceutical analysis play a crucial role in drug safety and compliance. By identifying trace impurities within permissible limits, these tests prevent toxicity, ensure quality control, and meet regulatory guidelines. Advanced spectroscopic techniques further enhance detection precision, making pharmaceutical analysis more reliable.
Ensuring rigorous limit testing in pharmaceuticals safeguards public health, environmental safety, and drug efficacy.