Rising prescription drug prices have remained one of the most persistent challenges in the United States healthcare system. Despite being a global leader in pharmaceutical innovation, the U.S. continues to face criticism for high medication costs, limited price transparency, and unequal access to essential therapies. Over the past decade, policymakers have explored multiple strategies to control drug spending while preserving incentives for innovation.
Against this backdrop, TrumpRx has emerged as a government-backed prescription drug discount initiative aimed at improving the affordability of medicines for American patients. The program represents a policy-driven intervention rather than a purely market-based discount model, distinguishing it from traditional coupon or pharmacy-driven platforms.
This article provides a detailed and professional analysis of the TrumpRx program, focusing on its structure, regulatory framework, operational mechanism, and broader implications for the pharmaceutical industry, global drug pricing, and pharma careers.
Overview of the TrumpRx Program
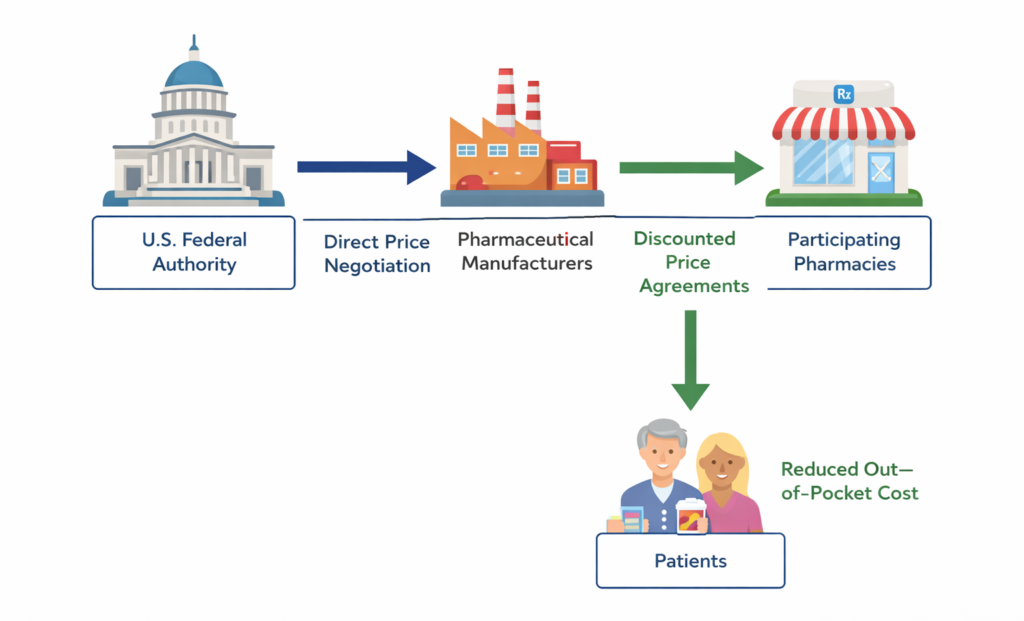
TrumpRx is designed as a centralized prescription drug discount platform supported at the federal level. The primary objective of the program is to reduce out-of-pocket costs for patients by facilitating access to discounted prescription medicines, particularly high-cost branded therapies.
Unlike private discount cards or coupon-based platforms, TrumpRx positions itself as a policy-backed initiative with direct engagement between the government and pharmaceutical manufacturers. The program aims to leverage national-level negotiating power to secure lower prices, thereby addressing one of the core drivers of high healthcare expenditure in the U.S.
Key objectives of TrumpRx include:
- Improving the affordability of prescription drugs
- Enhancing access for seniors and uninsured populations
- Increasing price transparency in the pharmaceutical supply chain
- Reducing financial barriers to long-term therapy adherence
From a policy standpoint, TrumpRx reflects a growing global trend where governments play a more active role in drug price regulation and negotiation.
Regulatory and Policy Framework Behind TrumpRx
The regulatory foundation of TrumpRx is rooted in federal authority over healthcare cost containment and drug pricing oversight. While the U.S. traditionally relies on market competition rather than direct price controls, escalating drug costs have pushed policymakers toward more interventionist approaches.
TrumpRx aligns with broader healthcare policy goals such as:
- Cost containment within public healthcare programs
- Reduction of patient-level financial toxicity
- Encouragement of value-based pricing models
The program operates within existing regulatory structures governing prescription drug distribution, pharmacy benefit management, and manufacturer compliance. Importantly, TrumpRx does not replace Medicare or private insurance plans but rather serves as a complementary pricing mechanism.
Policy discussions surrounding TrumpRx also intersect with debates on international reference pricing, generic competition, and the balance between affordability and innovation.
Operational Mechanism of TrumpRx
1. Drug Price Negotiation Strategy
At the core of TrumpRx is a centralized drug price-negotiation model. The program enables direct engagement with pharmaceutical manufacturers to secure discounted prices on selected prescription medicines.
This negotiation strategy focuses primarily on:
- Branded drugs with high retail prices
- Therapies used in chronic and long-term conditions
- Medicines with limited generic competition
By aggregating demand and leveraging federal backing, TrumpRx seeks to create pricing pressure that individual patients or pharmacies cannot achieve independently. This model mirrors negotiation approaches used in several European healthcare systems, though adapted to the U.S. regulatory environment.
2. Distribution and Access Model
TrumpRx is structured as a digital-first platform that connects patients to participating pharmacies. Eligible users can access discounted prices through the official platform, which serves as a centralized interface for price information and availability.
Key features of the access model include:
- Online price visibility
- Pharmacy-level fulfillment
- Simplified patient enrollment
This approach minimizes administrative complexity while maintaining compatibility with existing pharmacy infrastructure.
Therapeutic Categories Likely to Be Covered
While the complete list of drugs included under TrumpRx may evolve, the program is expected to prioritize therapeutic areas associated with high patient burden and significant healthcare costs.
Likely categories include:
- Diabetes and metabolic disorders (insulin and oral antidiabetics)
- Cardiovascular diseases (antihypertensives, anticoagulants)
- Oncology and specialty medicines
- Respiratory and autoimmune disorders
These categories represent areas where drug costs are a major contributor to patient non-adherence and long-term complications.
Compared to Medicare Part D, TrumpRx may offer additional pricing flexibility, particularly for patients who fall outside traditional coverage frameworks.
Comparative Assessment of Drug Discount Models
TrumpRx vs Market-Based Discount Platforms
Traditional discount platforms such as GoodRx or BlinkRx operate primarily through coupon aggregation and pharmacy-specific pricing agreements. These models rely on market competition rather than policy-driven negotiation.
Key differences include:
- Government involvement: TrumpRx benefits from federal backing, while private platforms do not
- Negotiation approach: Direct manufacturer engagement versus coupon-based discounts
- Pricing sustainability: Policy-backed pricing may offer more consistent discounts
From an industry perspective, TrumpRx introduces a structural shift by integrating public-sector influence into drug discount mechanisms.
Implications for the U.S. Pharmaceutical Industry
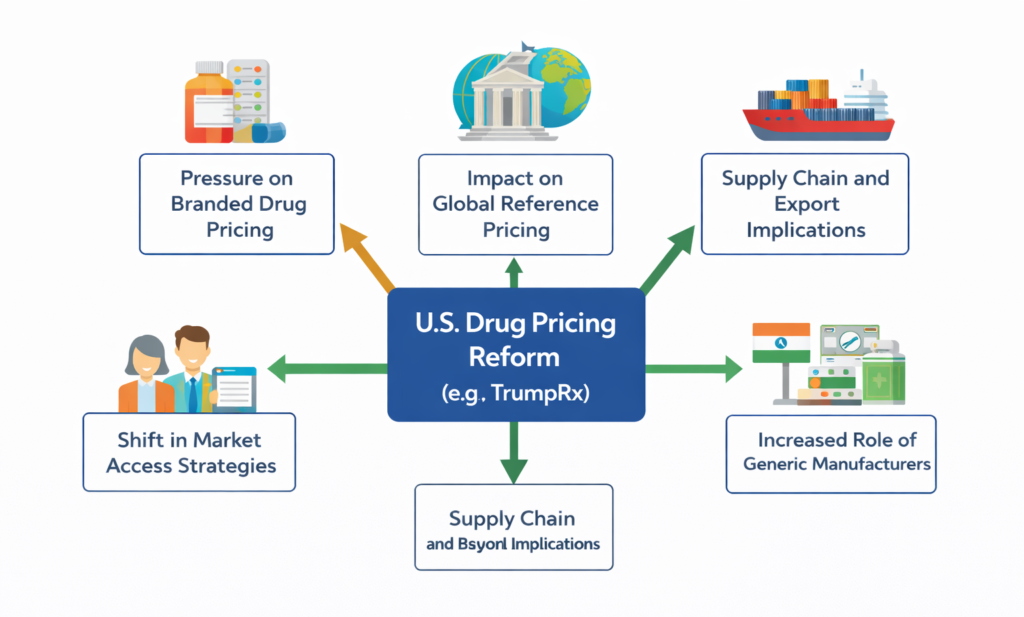
The introduction of TrumpRx has significant implications for pharmaceutical manufacturers operating in the U.S. market. Drug pricing strategies, particularly for branded therapies, may face increased scrutiny and pressure.
Potential industry impacts include:
- Reduced pricing flexibility for high-cost drugs
- Greater emphasis on value demonstration and outcomes-based pricing
- Increased focus on market access and reimbursement strategy
Manufacturers may need to reassess launch prices, lifecycle management plans, and payer engagement models to remain competitive under evolving pricing frameworks.
While revenue pressures are a concern, companies that proactively adapt to policy-driven pricing environments may benefit from improved market access and volume stability.
Global and Supply Chain Implications
U.S. drug prices often serve as a reference point for global pharmaceutical markets. As a result, initiatives such as TrumpRx may have downstream effects beyond national borders.
Potential global implications include:
- Influence on international reference pricing systems
- Increased pressure on multinational pricing alignment
- Opportunities for generic manufacturers, particularly from India
For Indian pharmaceutical companies, changes in U.S. pricing dynamics could affect export strategies, contract manufacturing opportunities, and regulatory planning.
Relevance of TrumpRx for Pharmaceutical Careers
1. Emerging Functional Roles
Policy-driven pricing reforms such as TrumpRx are reshaping workforce requirements across the pharmaceutical sector. Professionals with expertise at the intersection of policy, economics, and market strategy are increasingly in demand.
Key roles likely to gain prominence include:
- Market access and pricing analysts
- Health economics and outcomes research (HEOR) specialists
- Regulatory affairs and policy advisory professionals
These roles play a critical part in navigating complex pricing environments and ensuring compliance with evolving regulations.
2. Skills and Competencies in Demand
Pharma professionals seeking to future-proof their careers should focus on developing skills aligned with policy-driven healthcare models, including:
- Drug pricing and reimbursement strategy
- Real-world evidence generation
- Regulatory intelligence and policy analysis
TrumpRx highlights the growing importance of non-traditional pharma career paths that extend beyond research and manufacturing.
Limitations and Implementation Challenges
Despite its potential benefits, TrumpRx faces several implementation challenges that may influence its long-term effectiveness.
Key limitations include:
- Manufacturer participation and compliance levels
- Administrative coordination across pharmacies
- Policy continuity across political cycles
Balancing affordability with innovation incentives remains a central challenge for all pricing reform initiatives.
Conclusion
TrumpRx represents a structured policy approach to addressing prescription drug affordability in the United States. By combining federal oversight with direct manufacturer engagement, the program seeks to reduce patient costs while maintaining supply chain integrity.
For the pharmaceutical industry, TrumpRx signals a shift toward greater policy involvement in pricing decisions. For professionals and students, it underscores the growing relevance of market access, health economics, and regulatory strategy as core career domains.
As drug pricing reforms continue to evolve, initiatives like TrumpRx will play a crucial role in shaping the future of healthcare affordability and pharmaceutical workforce planning.
Frequently Asked Questions (FAQ)
What is the primary objective of TrumpRx?
To reduce prescription drug costs through government-backed price negotiation.
How does TrumpRx differ from private discount platforms?
It involves direct policy-level engagement with manufacturers rather than coupon-based discounts.
Can TrumpRx influence drug pricing outside the U.S.?
Yes, due to the U.S. market’s global pricing influence.
Which pharma careers are most impacted by TrumpRx?
Market access, HEOR, regulatory affairs, and pricing strategy roles.



