The synthesis of 2,4,5-triphenyl imidazole is a classic example of multi-component organic reactions involving the condensation of benzil, benzaldehyde, and ammonium acetate. Imidazole and its derivatives play an important role in medicinal chemistry due to their wide spectrum of pharmacological activities, such as anti-cancer, antimicrobial, anti-inflammatory, and analgesic effects. This experiment teaches practical organic synthesis techniques like refluxing and recrystallization, and provides a deeper understanding of heterocyclic compound formation, especially of the biologically important imidazole ring. Students gain insights into reaction mechanisms, yield estimation, and compound characterization through this lab work.

Aim: To prepare and submit triphenyl imidazole (2,4,5-triphenyl imidazole).
References:
- Vogel’s Textbook of Practical Organic Chemistry by Brian S. Furniss, Antony J. Hannaford, Peter W. G. Smith & Austin R. Tatchell; Fifth Edition; Page No. 1193.
Requirements:
- Chemicals: benzil, ammonium acetate, benzaldehyde, and ethanol.
- Apparatus: reflux condenser, round-bottom flask, mechanical stirrer, measuring cylinder, funnel, and glass rod.
Principle:
Triphenyl imidazole was synthesized by condensing benzil, benzaldehyde, and ammonium acetate in an acetic medium. Imidazole (C3H4N2) and its derivatives show various pharmacological activities like antifungal and antibacterial, anti-inflammatory, analgesic, antitubercular, antidepressant, anticancer, and antiviral activity. Imidazole is put into multiple essential organic molecules. The most significant is the amino acid histidine, which has an imidazole side chain. Histidine is present in many proteins and enzymes and is vital to hemoglobin structure and binding functions. Later, histidine would decarboxylate to histamine. Triphenyl imidazole was synthesized by condensing benzil, benzaldehyde, and ammonium acetate in an acetic medium.
- Reaction:
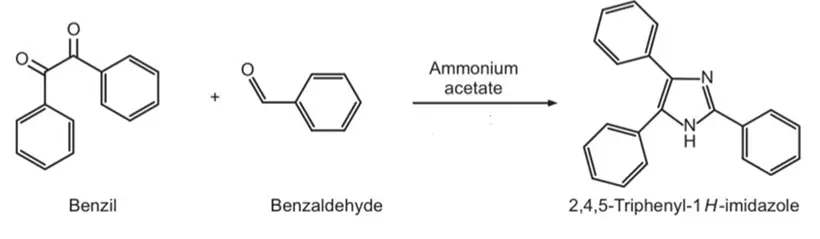
- Use: Anti-cancer, anti-microbial, anti-inflammatory, anti-tubercular, and analgesic.
Procedure:
- Place 1.2 g of benzil, 20 ml of benzaldehyde, and 1.27 g of ammonium acetate into a 250 ml round-bottom flask equipped with a magnetic stirrer. Heat the mixture in a water bath at 100°C for 4 hours while stirring continuously.
- Once the reaction is complete, wash the mixture with ice-cold water, then recrystallize the solid crude product from ethanol.
- After recrystallization, dry the product thoroughly. Calculate the percentage yield and determine its melting point.
Calculation:
The limiting reagent is benzil; Hence, the yield should be calculated from the amount taken.
Molecular formula of benzil = C14H10O2
The molecular formula of 2,4,5-triphenyl imidazole = C21H16N2
Molecular weight of benzil = 210 g/mole
And molecular weight of 2,4,5-triphenyl imidazole = 296 g/mole
Theoretical yield:
210g benzil forms 296 g 2,4,5-triphenyl imidazole
Therefore, 1.2 g benzil will form …….? (X) g 2,4,5-triphenyl imidazole
296 X 1.2/210= 1.69 g
Theoretical yield = 1.69 g
Practical yield = ————- g
% Yield = (Practical Yield)/(Theoretical Yield) × 100
Result:
2,4,5-triphenyl imidazole was synthesized from benzil and submitted.
| Name of Compound | 2,4,5-triphenyl imidazole |
| Theoretical yield | ……gm |
| Practical yield | ……gm |
| % Practical yield | .……% |
| Melting point | .……ͦC |
How to Get Your Practical PDF Copy:
- Click the download link below.
- Dive into a wealth of knowledge and elevate your learning experience!
Frequently Asked Questions (FAQs)
1. What is the use of 2,4,5-triphenyl imidazole in medicinal chemistry?
Triphenyl imidazole exhibits anti-cancer, anti-inflammatory, antimicrobial, and analgesic properties, making it a valuable compound in drug discovery.
2. How is 2,4,5-triphenyl imidazole synthesized in the lab?
It is synthesized by condensing benzil, benzaldehyde, and ammonium acetate under heating in an acetic medium, followed by recrystallization.
3. Why is benzil considered the limiting reagent in this experiment?
Benzil is used in the smallest molar quantity and determines the maximum amount of product that can be formed.
4. What is the mechanism of triphenyl imidazole formation?
The reaction involves nucleophilic addition of ammonia to carbonyl groups, followed by cyclization and aromatization to form the imidazole ring.
5. What is the melting point of 2,4,5-triphenyl imidazole?
The pure compound has a melting point around 270–275°C, although it should be confirmed in the practical based on the sample.