Quantitative Structure-Activity Relationship (QSAR) is a method that helps researchers understand the link between a chemical compound’s structure and its biological activity. One of the most essential parts of QSAR studies involves using physicochemical parameters to predict how a compound will behave in a biological system. These parameters include partition coefficients, electronic properties, and steric effects. In this article, we will look at the most commonly used physicochemical parameters in QSAR: the partition coefficient, Hammett’s electronic parameter, Taft’s steric parameter, and Hansch analysis.
What is QSAR?
QSAR is used in drug discovery, toxicology, and environmental chemistry to predict how chemical compounds affect biological systems. The foundation of QSAR is that similar molecules often have similar properties. By quantifying structural features, researchers can forecast biological activity, which reduces time and cost in drug development.
Importance of Physicochemical Parameters in QSAR
Physicochemical parameters describe how a molecule interacts with its surroundings. These include:
- Lipophilicity (fat solubility)
- Electronic effects
- Steric hindrance (molecular size/shape)
- Hydrogen bonding ability
Each of these can influence how a drug is absorbed, distributed, metabolized, or excreted (ADME properties) and ultimately how it binds to its biological target.
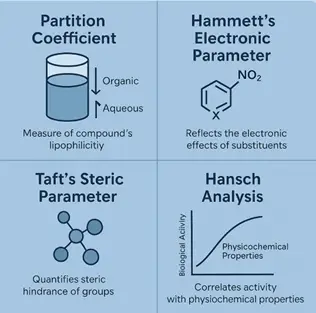
Figure: Overview of key physicochemical parameters used in QSAR studies.
This infographic visually summarizes the key physicochemical parameters used in QSAR and their roles in drug design and prediction.
Partition Coefficient (Log P)
The partition coefficient, commonly denoted as Log P, measures how a compound distributes itself between a hydrophobic phase (like octanol) and a hydrophilic phase (like water). It indicates how lipophilic (fat-loving) or hydrophilic (water-loving) a compound is.
Why it matters:
- Lipophilicity affects drug absorption, membrane permeability, and distribution.
- Compounds with very high log P may accumulate in fatty tissues and show toxicity.
- Very low log P values may indicate poor cell membrane penetration.
Typical range for drug candidates:
- Log P between 1 and 3 is usually optimal.
Example:
- Caffeine has a Log P of -0.07 (hydrophilic)
- Diazepam has a Log P of 2.82 (moderately lipophilic)
Hammett’s Electronic Parameter (σ)
Hammett’s constant (σ) is used to measure the electronic effect of substituents on aromatic rings. It indicates whether a group donates or withdraws electrons.
Why it matters:
- Electronic effects influence reactivity, stability, and binding to enzymes or receptors.
- Electron-withdrawing groups (e.g., -NO2) can increase acidity.
- Electron-donating groups (e.g., -OH, -NH2) can stabilize positive charges.
How it’s used:
- In QSAR, σ values are correlated with activity to find the best functional groups.
Example:
- σ for -NO2 is +0.78 (strong electron-withdrawing)
- σ for -OCH3 is -0.27 (electron-donating)
Taft’s Steric Parameter (Es)
Taft’s steric parameter (Es) measures the spatial effect of substituents, specifically their bulkiness or size. It helps in assessing how much a substituent hinders the interaction of a molecule with its target.
Why it matters:
- Larger groups can prevent a molecule from fitting into a receptor site.
- Optimal steric balance can improve binding and selectivity.
How it’s measured:
- Derived from the effect of substituents on reaction rates in aliphatic systems.
Example:
- -CH3 has Es = 0.00
- -C(CH3)3 (tert-butyl) has Es = -3.12 (bulky)
Hansch Analysis
Hansch analysis is a statistical method that combines various physicochemical parameters to correlate molecular features with biological activity. It often uses regression models.
Why it matters:
- Helps in building predictive models.
- Identifies which properties contribute most to activity.
- Guides the design of better drugs.
Typical equation:
Biological activity = a(Log P) + b(σ) + c(Es) + d
Where:
- a, b, c = coefficients showing how strongly each parameter affects activity.
- d = intercept.
Applications:
- Used widely in anticancer, antimicrobial, and anti-inflammatory drug design.
Combined Use of Physicochemical Parameters in Drug Discovery
In modern QSAR studies, no single parameter gives a complete picture. Combinations of Log P, σ, Es, and other descriptors are used to build more accurate models.
Software and Tools:
- ChemOffice
- MOE (Molecular Operating Environment)
- QSARINS
- Python and R (for statistical modeling)
Challenges and Limitations of Physicochemical QSAR
Despite its usefulness, QSAR based on physicochemical parameters has some limitations:
- Biological activity is multifactorial—parameters alone may not capture complexity.
- Models depend on the quality and size of data sets.
- Outliers can distort statistical conclusions.
How to overcome them:
- Use 3D-QSAR models (e.g., CoMFA, CoMSIA)
- Validate models with external test sets.
- Integrate with machine learning algorithms.
Future Trends in QSAR and Drug Design
QSAR is evolving with the integration of AI, machine learning, and big data analytics. These tools can:
- Handle large chemical libraries.
- Improve prediction accuracy.
- Identify patterns that human experts may miss.
Other advanced techniques:
- Molecular docking
- Pharmacophore modeling
- Deep learning QSAR (DL-QSAR)
Conclusion
Physicochemical parameters like Log P, Hammett’s electronic constant, Taft’s steric parameter, and Hansch analysis form the backbone of classical QSAR. These descriptors help scientists predict biological activity and optimize drug candidates. With the help of statistical tools and computational software, QSAR models are becoming more reliable and insightful. As drug design advances, combining these traditional parameters with modern machine learning techniques will unlock more precise and efficient drug discovery pathways.
Frequently Asked Questions (FAQs)
1: What are the physicochemical parameters in QSAR?
Answer: Physicochemical parameters in QSAR are measurable properties of molecules like lipophilicity, electronic effects, and steric factors that help predict how a chemical structure influences biological activity. Common examples include the partition coefficient (log P), Hammett’s constant (σ), and Taft’s steric parameter (Es).
Q 2: Why is the partition coefficient important in QSAR studies?
Answer: The partition coefficient (log P) indicates how a compound distributes between water and lipid environments. It helps assess drug absorption and bioavailability. Compounds with optimal log P values are more likely to cross biological membranes and reach their target effectively.
Q 3: What is the role of Hammett’s electronic parameter in QSAR?
Answer: Hammett’s electronic parameter (σ) measures the effect of substituents on the electronic properties of a molecule. It is especially useful for understanding how electron-withdrawing or donating groups influence reactivity, which affects how well a drug interacts with its target.
Q 4: How does Hansch analysis help in drug discovery?
Answer: Hansch analysis combines multiple physicochemical parameters, like log P, σ, and Es, into a mathematical model to correlate with biological activity. It helps researchers predict how modifying a molecule’s structure can improve or reduce its drug-like behavior.
Q 5: Can QSAR be used without knowing the target protein?
Answer: Yes. QSAR is a ligand-based approach that relies on the properties of known active molecules rather than the target’s structure. It is particularly useful when the biological target is unknown or not well characterized.



