1,3-Diphenyl pyrazole is a heterocyclic compound featuring a five-membered pyrazole ring substituted with phenyl groups at the 1 and 3 positions. Pyrazole derivatives are well-known for their diverse biological activities, including anti-inflammatory, analgesic, antimicrobial, and anticancer properties. In this practical, 1,3-diphenyl pyrazole is synthesized via the reaction of diphenyl hydrazone with a vicinal diol, typically under acidic or dehydrating conditions. This experiment demonstrates fundamental principles of organic synthesis, such as cyclization and condensation, and helps students understand the reactivity of hydrazones and diols in forming heterocyclic structures.
Aim: To prepare and submit 1,3-diphenyl pyrazole from diphenyl hydrazone and a vicinal diol
References:
- Practical Heterocyclic Chemistry by A. O. Fitton and R. K. Smalley, Academic Press, London and New York, pages 25-26.
Requirements:
Chemicals: 1-benzyledene-2-phenyl hydrazine, ethane-1,2-diol (ethylene glycol), ferric chloride, tert-butyl hydroperoxide, acetylacetone, sodium chloride, ethyl acetate, and sodium sulfate.
Apparatus: Water bath, Beaker, Measuring cylinder, Thermometer, Stirrer, Separatory funnel, Buchner funnel, etc.
Principle:
1,3-substituted pyrazole is prepared by cyclization of diaryl hydrazone and vicinal diol in the presence of ferric chloride and tert-butyl hydroperoxide (TBHP), which is also called the regioselective synthesis of substituted pyrazole.
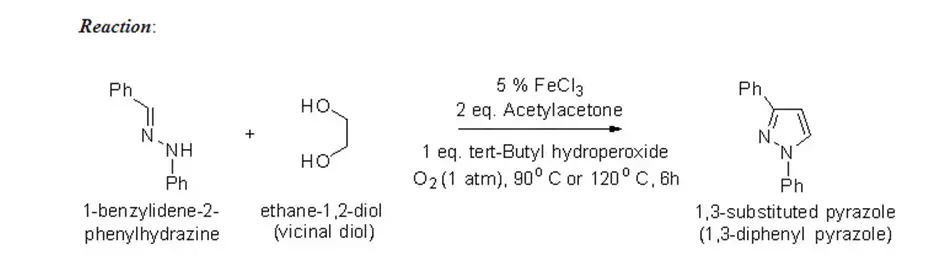
Procedure:
About 4.55 g of 1-benzyledene-2-phenyl hydrazine is dissolved in 25 ml of vicinal diol and ferric chloride (5 mol% ). Then, another solution of tert-butyl hydroperoxide (5.3 g) in 25 mL of acetylacetone is added. The mixed solution is maintained at a temperature range of 90 to 100°C.
The mixed solution is left to reach room temperature and stirred for 6 hours. Content is poured into water and extracted with ethyl acetate three times. The combined organic solution is washed with water, then with a saturated solution of sodium chloride, passed through sodium sulfate, and evaporated under a vacuum. About 3.15 g of the final product is found with m.p. 185 °C.
Calculation:
– Molecular Formula of 1-benzyledene-2-phenyl hydrazine = C13H12N2
– MF of 1,3-diphenyl pyrazole = C15H12N2
– Molecular weight of 1-benzyledene-2-phenyl hydrazine = 196 g/ mol
– MW of 1,3-diphenyl pyrazole = 220 g/ mol
196 g of 1-benzyledene-2-phenyl hydrazine yields 1,3-diphenyl pyrazole = 220 g
4.55 g of 1-benzyledene-2-phenyl hydrazine shall yield 1,3-diphenyl pyrazole = (220 / 196) × 4.55 = 5.1 g
Therefore, the Theoretical yield of 1,3-diphenyl pyrazole = 5.1 g
If reported Practical yield = …..g
Then, Percentage Practical yield = (Practical yield / Theoretical yield) × 100
= (…. / 5.1) × 100 = …..%
Result:
The percent yield of 1,3-diphenyl pyrazole is …. % with m.p. …. °C.
How to Get Your Practical PDF Copy:
- Click the download link below.
- Dive into a wealth of knowledge and elevate your learning experience!
Frequently Asked Questions (FAQs)
1. What is the role of vicinal diol in the synthesis of 1,3-diphenyl pyrazole?
Vicinal diol serves as the carbon source for the pyrazole ring formation. It reacts with diphenyl hydrazone to initiate cyclization, forming the five-membered heterocycle.
2. Why is diphenyl hydrazone used as a starting material?
Diphenyl hydrazone provides the necessary nitrogen atoms for the pyrazole ring. It is reactive toward diols under acidic conditions, making it a suitable precursor for pyrazole synthesis.
3. What are the reaction conditions typically required for this synthesis?
The reaction usually requires heating under acidic conditions or a dehydrating agent to facilitate cyclization and eliminate water, aiding in ring closure.
4. How can the product (1,3-diphenyl pyrazole) be identified or confirmed?
The synthesized product can be identified using techniques such as melting point determination, thin-layer chromatography (TLC), and IR spectroscopy to confirm functional groups.
5. What are some potential applications of 1,3-diphenyl pyrazole and its derivatives?
1,3-Diphenyl pyrazole derivatives are studied for their pharmaceutical properties, including anti-inflammatory, analgesic, and antitumor activities. They are also used in agrochemicals and dye manufacturing.




