Pharmacophore modeling is an essential concept in drug discovery and medicinal chemistry. It helps scientists identify and design compounds likely to interact with a specific biological target. This SEO-optimized post aims to provide a complete and easy-to-understand explanation of pharmacophore modeling, its importance, how it works, and its wide range of applications.
What is a pharmacophore?
A pharmacophore is the set of structural features in a molecule that is recognized by a biological target (such as a receptor or enzyme) and is responsible for its biological activity. These features typically include hydrogen bond acceptors/donors, hydrophobic regions, aromatic rings, and charged groups.
The International Union of Pure and Applied Chemistry (IUPAC) defines a pharmacophore as:
“An ensemble of steric and electronic features that is necessary to ensure the optimal interactions with a specific biological target and to trigger (or block) its biological response.”
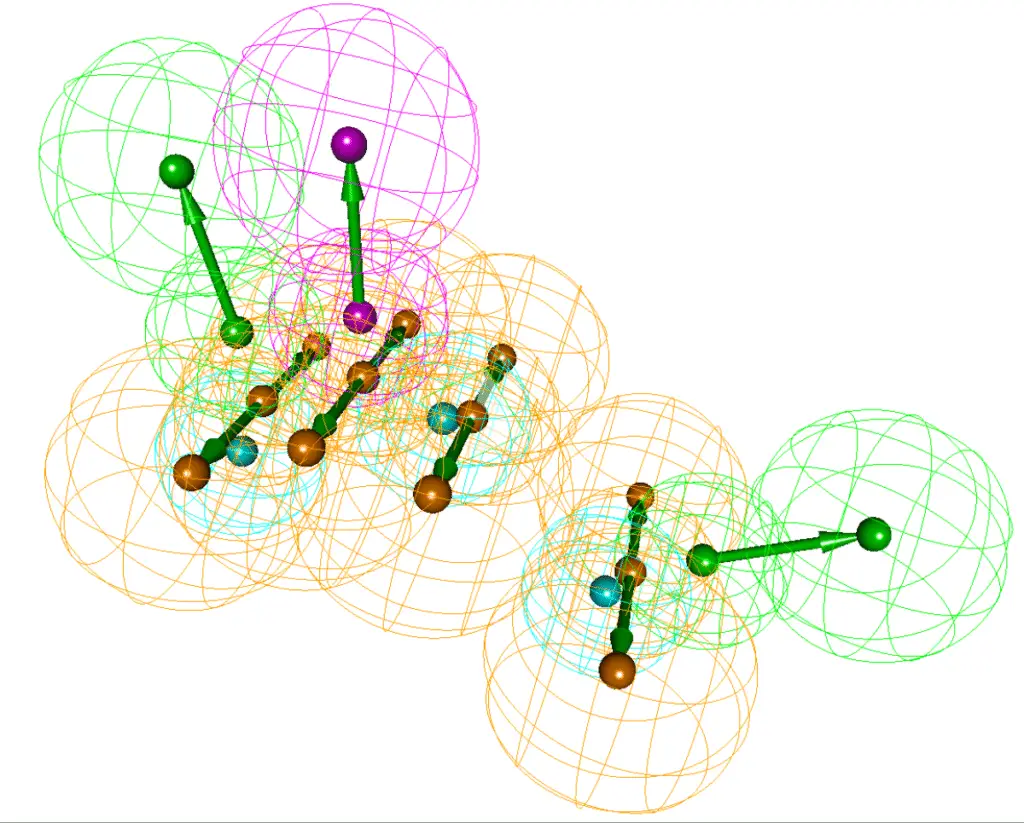
Why is pharmacophore modeling important?
Pharmacophore modeling plays a vital role in the early stages of drug development. It allows researchers to:
- Understand the essential interactions between a drug and its target
- Identify new active compounds
- Reduce the cost and time involved in experimental drug screening
- Guide the optimization of lead compounds
In essence, pharmacophore models are blueprints for drug discovery.
Key Elements of a Pharmacophore Model
- Hydrogen Bond Donors (HBD): Atoms or groups that donate a hydrogen bond (e.g., -OH, -NH2).
- Hydrogen Bond Acceptors (HBA): Atoms or groups that accept a hydrogen bond (e.g., carbonyl oxygen).
- Hydrophobic Centers: Non-polar regions that interact with hydrophobic pockets in the target.
- Aromatic Rings: Flat ring systems that participate in stacking interactions.
- Positive/Negative Ionizable Groups: Groups that can donate or accept a proton (e.g., NH3+, COO-).
Types of Pharmacophore Modeling
There are two main types of pharmacophore modeling:
1. Ligand-Based Pharmacophore Modeling
This method is used when the 3D structure of the biological target is not known. It relies on analyzing a set of known active compounds to identify common features.
2. Structure-Based Pharmacophore Modeling
This method uses the 3D structure of the target protein, often obtained through X-ray crystallography or cryo-electron microscopy. It allows direct identification of interaction points on the target.
Steps in Pharmacophore Modeling
Step 1: Data Collection
- Collect known active compounds.
- Gather structural data of the target (if available).
Step 2: Feature Identification
- Identify key pharmacophoric features in the active compounds.
- Use software tools to extract these features.
Step 3: Model Generation
- Generate a 3D pharmacophore model using tools like Catalyst, LigandScout, or MOE.
Step 4: Validation
- Validate the model by testing it against known active and inactive compounds.
- Evaluate the sensitivity and specificity.
Step 5: Virtual Screening
- Screen large compound libraries to find molecules that match the pharmacophore.
Step 6: Hit Selection and Optimization
- Select hits and optimize them for better activity, bioavailability, and reduced toxicity.
Software Tools for Pharmacophore Modeling
Some popular software platforms used include:
- LigandScout: Great for 3D pharmacophore modeling.
- Discovery Studio: Comprehensive tools for both ligand- and structure-based modeling.
- MOE (Molecular Operating Environment): Used for pharmacophore generation and visualization.
- Pharmit: Free web-based pharmacophore modeling and screening tool.
- ZINCPharmer: For searching the ZINC database using pharmacophore queries.
Applications of Pharmacophore Modeling
- Hit Identification: Identify potential lead molecules from large databases.
- Lead Optimization: Modify molecules to improve potency and reduce side effects.
- Target Validation: Understand which molecular features are essential for binding.
- Multi-Target Drug Design: Design drugs that interact with more than one target.
- Repurposing Existing Drugs: Find new uses for existing drugs based on pharmacophoric features.
Advantages of Pharmacophore Modeling
- Helps identify unknown bioactive compounds.
- Supports rational drug design.
- Reduces experimental workload.
- Can be used with limited structural information.
- Works well in combination with other in silico methods like QSAR and molecular docking.
Limitations of Pharmacophore Modeling
- Depends on the quality and diversity of input data.
- It may not capture the dynamic flexibility of molecules.
- Can produce false positives/negatives during screening.
- Limited if the binding mode is not conserved across molecules.
Future Directions
Pharmacophore modeling is evolving rapidly with the integration of artificial intelligence and machine learning. Future advancements will focus on:
- AI-based feature recognition
- Integration with omics data (genomics, proteomics)
- Personalized drug design using patient-specific models
- Real-time pharmacophore modeling using cloud platforms
Conclusion
Pharmacophore modeling is a key technique in modern drug discovery. By understanding the necessary features for drug-target interactions, researchers can accelerate the identification of promising candidates, improve drug design, and ultimately enhance patient outcomes. As computational tools and AI evolve, pharmacophore modeling will continue to play a vital role in shaping the future of precision medicine.
Frequently Asked Questions (FAQs)
1. What is pharmacophore modeling, and why is it important in drug discovery?
Answer: Pharmacophore modeling is a computational technique used to identify the essential features of molecules that interact with a specific biological target. These features include hydrogen bond donors or acceptors, hydrophobic regions, and aromatic rings. By understanding these key interactions, researchers can design or identify new compounds that may serve as effective drugs. This approach streamlines the drug discovery process by focusing on compounds with the desired biological activity.
2. What are the main types of pharmacophore modeling?
Answer: There are two primary types of pharmacophore modeling:
- Ligand-Based Pharmacophore Modeling: This method relies on analyzing a set of known active compounds to identify common features responsible for their activity. It’s particularly useful when the 3D structure of the target protein is unknown.
- Structure-Based Pharmacophore Modeling: This approach utilizes the 3D structure of the target protein to identify potential interaction sites. It allows for the design of compounds that can specifically bind to the target’s active site.
3. How does pharmacophore modeling differ from molecular docking?
Answer: While both pharmacophore modeling and molecular docking are used in drug discovery, they serve different purposes:
- Pharmacophore Modeling: Focuses on identifying the essential features required for molecular recognition and activity, often used for virtual screening of large compound libraries.
- Molecular Docking: Simulates the interaction between a molecule and its target protein to predict the binding affinity and orientation. It’s typically used to refine and validate hits identified through pharmacophore modeling.
Combining both methods can enhance the efficiency and accuracy of the drug discovery process.
4. What are the limitations of pharmacophore modeling?
Answer: Despite its advantages, pharmacophore modeling has some limitations:
- Dependence on Quality Data: The accuracy of the model heavily relies on the quality and diversity of the input data.
- Static Representation: Traditional pharmacophore models may not account for the dynamic nature of molecular interactions.
- False Positives/Negatives: There’s a risk of identifying compounds that fit the model but lack actual biological activity, or missing active compounds that don’t fit the model perfectly.
To mitigate these issues, pharmacophore modeling is often used in conjunction with other computational and experimental methods.
5. Which software tools are commonly used for pharmacophore modeling?
Answer: Several software tools are widely used in pharmacophore modeling, including:
- LigandScout: Offers intuitive 3D pharmacophore modeling and visualization.
- Discovery Studio: Provides comprehensive tools for both ligand- and structure-based modeling.
- MOE (Molecular Operating Environment): Used for pharmacophore generation, visualization, and virtual screening.
- Pharmit: A free web-based platform for pharmacophore modeling and screening.
- ZINCPharmer: Allows users to search the ZINC database using pharmacophore queries.
These tools assist researchers in identifying and optimizing potential drug candidates efficiently.




