Drug design is one of the most exciting and fast-growing areas in the pharmaceutical sciences. It plays a central role in the discovery and development of new medicines. In this blog post, we will look at what drug design is, its significance in healthcare, and the various scientific approaches used to create effective drugs.
What is Drug Design?
Drug design is the process of finding new medications based on the knowledge of a biological target. It involves creating molecules that interact with specific proteins or genes in the human body to prevent or treat diseases.
Instead of discovering new drugs by trial and error, drug design uses a more targeted and systematic approach. It combines chemistry, biology, and computer science to develop new compounds with potential therapeutic benefits.
Importance of Drug Design in Modern Medicine
Drug design helps in:
- Reducing the time and cost of drug development
- Increasing the chances of drug success
- Designing safer and more effective drugs
- Targeting diseases at the molecular level
- Personalizing treatment based on patient-specific targets
Drug design has revolutionized the way we treat conditions such as cancer, HIV/AIDS, Alzheimer’s, diabetes, and infectious diseases.
Basic Concepts in Drug Design
Understanding the core concepts of drug design is essential for anyone interested in how new medicines are developed. Before a drug can be created, scientists must understand how molecules interact with biological systems. Below are four fundamental terms you should know in the field of drug discovery and design.
a. What is a Drug Target in Drug Design?
A drug target is typically a biological molecule, such as a protein, enzyme, receptor, or DNA, that plays a critical role in the development or progression of a disease. Drug design efforts are focused on developing compounds that can interact with these targets to modify disease activity.
Examples of drug targets:
- G-protein-coupled receptors (GPCRs)
- Ion channels
- Kinase enzymes
- Nucleic acids (e.g., in cancer or viral diseases)
Identifying the right drug target is often the first step in modern drug discovery.
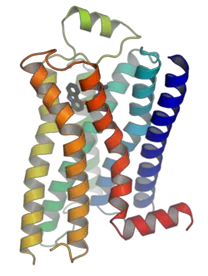
Figure: Drug target of G-protein-coupled receptors (GPCRs) (Source: Wikipedia)
b. What is a Ligand in Drug Design?
A ligand is a chemical compound or molecule that binds to a drug target to trigger a biological effect. Ligands can act as:
- Agonists, which activate the target
- Antagonists, which block the target
In drug design, selecting or designing the right ligand is crucial because it determines the therapeutic outcome. The ligand’s shape, charge, and chemical properties must complement the target’s binding site.
c. What is a Binding Site in Drug Design?
The binding site is the specific location on a target molecule, usually a protein, where the ligand binds. This region is often a pocket or groove that fits the ligand like a key in a lock.
Binding sites can contain:
- Hydrophobic regions
- Hydrogen bond acceptors and donors
- Charged or polar amino acids
Understanding the structure of the binding site helps in rational drug design, allowing for more accurate molecular modeling and prediction of binding affinity.
d. Affinity and Selectivity in Drug Design
Affinity refers to the strength of the interaction between a ligand and its drug target. Higher affinity means the ligand binds more tightly and is likely to be more effective at lower doses.
Selectivity means the drug binds primarily to the intended target and not to other proteins, reducing the risk of side effects or off-target toxicity.
Why is selectivity important?
- Prevents unwanted reactions
- Enhances drug safety
- Increases treatment specificity
Improving affinity and selectivity is a key focus during the optimization stage of drug development.
Key Stages of Drug Design
- Target identification and validation
- Lead compound identification
- Lead optimization
- Preclinical testing
- Clinical trials
Each of these steps involves advanced tools, testing, and evaluation to ensure the safety and effectiveness of the designed drug.
Drug design is a step-by-step process that transforms a basic scientific idea into a safe and effective medication. Each stage involves research, testing, and careful evaluation to improve the chances of developing a successful drug. Below are the five major stages:
1. Target Identification and Validation
The first step in drug design is identifying a biological target, usually a protein, enzyme, or gene, that plays a critical role in a disease. Once identified, scientists must validate the target to confirm that modifying it (inhibiting or activating) can beneficially influence the disease. This stage often uses genomics, proteomics, and bioinformatics tools to find disease-specific targets.
2. Lead Compound Identification
After choosing a target, researchers search for a lead compound molecule that shows potential to interact with the target effectively. This can be done through:
- High-throughput screening of chemical libraries,
- Natural product screening, or
- Structure-based design using computational tools.
The goal here is to find a compound that binds to the target and produces the desired biological effect.
3. Lead Optimization
Once a lead compound is found, it undergoes chemical modifications to improve its properties. This includes:
- Enhancing potency (stronger effect),
- Improving selectivity (fewer side effects),
- Increasing bioavailability (how well it works in the body), and
- Reducing toxicity.
Medicinal chemists use structure–activity relationships (SAR) and computational modeling to guide this optimization process.
4. Preclinical Testing
In this stage, the optimized compound is tested in non-human models (such as cell cultures and animals) to evaluate:
- Pharmacokinetics (how the drug moves through the body),
- Pharmacodynamics (how the drug affects the body),
- Toxicity, and
- Dosage requirements.
The goal is to ensure the drug is safe and effective enough to proceed to human trials.
5. Clinical Trials
After successful preclinical testing, the drug enters clinical trials, which are conducted in three main phases:
- Phase I: Tests safety and dosage in a small group of healthy volunteers.
- Phase II: Assesses efficacy and side effects in a larger group of patients.
- Phase III: Confirms effectiveness, monitors side effects, and compares the drug to standard treatments in a large population.
If successful, the drug can then be submitted for regulatory approval (e.g., FDA or EMA) before being released to the market. Each stage in the drug design process builds on the previous one. From identifying the right target to testing the final product in humans, the entire pipeline is designed to ensure that new drugs are safe, effective, and precisely tailored to treat specific diseases. These stages are vital for developing modern therapies and are increasingly supported by artificial intelligence, machine learning, and computational chemistry tools.
Types of Drug Design
Drug design refers to the process of discovering or creating new medicines to treat diseases. There are several approaches that scientists use, and each has its strengths depending on the disease, target, and available knowledge.
Below are the four major types of drug design used in pharmaceutical research:
1 Rational Drug Design: Target-Based Drug Discovery
Rational drug design is a scientific approach based on the detailed knowledge of the biological target’s structure, such as a protein or enzyme. In this method, scientists use 3D models of the target to design a drug molecule that fits precisely into the active site.
Key features:
- Requires information about the target’s molecular structure (often from X-ray crystallography or NMR).
- Involves structure-based drug design (SBDD) and ligand-based drug design (LBDD).
- Uses computer-aided tools like molecular docking and virtual screening.
Example: Designing HIV protease inhibitors by analyzing the structure of the HIV protease enzyme.
2 Random Screening in Drug Discovery
Random screening involves testing thousands of chemical compounds on biological systems to observe if any of them have desirable effects without any prior knowledge of the target.
Key features:
- High-throughput screening (HTS) is often used.
- Hits (active compounds) are identified first, and their targets are studied later.
- This method has historically led to many drug discoveries, especially when little was known about the disease.
Example: Discovery of penicillin from Penicillium notatum by chance observation.
3 Chemical Modification of Existing Drugs
This approach involves modifying the structure of a known drug to enhance its efficacy, safety, stability, or absorption.
Key features:
- Based on known pharmacophores (active portions of a molecule).
- Small changes in chemical structure can significantly alter biological activity.
- Helps develop next-generation drugs from existing ones.
Example: Making doxycycline from tetracycline to improve oral absorption and reduce side effects.
4 Natural Product-Based Drug Design
Nature has always been a rich source of medicines. In natural product-based drug design, researchers use compounds derived from plants, microbes, or marine organisms as templates to develop new drugs.
Key features:
- Many natural products have complex structures that interact well with human proteins.
- Often serve as inspiration for semi-synthetic or fully synthetic analogs.
- Used in cancer, infectious disease, and pain treatment, drug discovery.
Example: Paclitaxel (Taxol) was derived from the bark of the Pacific yew tree and is used to treat cancer.
Table: A Summary of types of drug design
| Type | Based On | Tools Used | Example |
| Rational Drug Design | Target structure knowledge | Molecular docking, modeling | HIV protease inhibitors |
| Random Screening | No prior target knowledge | High-throughput screening | Penicillin |
| Chemical Modification | Existing drug molecules | Medicinal chemistry tools | Doxycycline from tetracycline |
| Natural Product-Based | Plant or microbial sources | Isolation, semi-synthesis | Paclitaxel (Taxol) |
Why Understanding These Drug Design Types Matters
Knowing the types of drug design helps researchers choose the most effective method based on:
- The availability of biological information
- The nature of the disease
- The resources and tools at hand
Whether starting from scratch or building on nature’s blueprints, each method plays a vital role in modern drug discovery and development.
Various Approaches Used in Drug Design
1. Ligand-Based Drug Design (LBDD)
Ligand-based drug design uses information about molecules that are known to interact with the target. If we know the structure of a few active compounds, we can use this data to design similar drugs.
Key techniques:
- Quantitative Structure-Activity Relationship (QSAR)
- Molecular similarity analysis
2. Structure-Based Drug Design (SBDD)
In structure-based drug design, scientists use the 3D structure of the target protein to design drugs that will fit perfectly into its active site, like a key in a lock.
Tools used:
- X-ray crystallography
- Molecular docking
- Homology modeling
3. Fragment-Based Drug Design (FBDD)
This approach starts with identifying small chemical fragments that bind weakly to the target. These fragments are then combined or modified to create a more powerful drug.
Benefits:
- Allows the discovery of novel binding sites
- Good for hard-to-drug targets
4. Computer-Aided Drug Design (CADD)
CADD is a powerful tool that uses software and simulations to predict how a drug will interact with its target. It helps in:
- Predicting drug-receptor interactions
- Optimizing lead compounds
- Reducing the need for lab experiments
Examples:
- Molecular dynamics
- Virtual screening
- Molecular docking
5. De Novo Drug Design
This approach involves designing drug molecules from scratch, without starting from existing drugs or fragments. Using algorithms, new molecules are built based on the shape and features of the target site.
Advantages:
- Unique structures
- Potentially better patent protection
6. Pharmacophore Modeling
A pharmacophore is an abstract description of molecular features necessary for drug activity. These features include hydrogen bond donors/acceptors, hydrophobic centers, and charged groups.
This method helps identify and design new molecules that meet these essential features.
7. Target-Oriented Drug Design
This method focuses on modifying the structure of the drug to increase its activity toward a specific biological target. It uses feedback from biological assays to refine the structure of the lead compound.
8. Combinatorial Chemistry Approach
This involves creating a large library of different molecules and testing them quickly to identify active candidates. It speeds up the lead discovery process and is often combined with high-throughput screening.
Challenges in Drug Design
Despite technological advances, drug design still faces several challenges:
- Drug Resistance: Especially in cancer and antibiotics.
- Target Validation: Ensuring the target is actually involved in the disease.
- Side Effects: Off-target interactions may cause unwanted effects.
- Bioavailability: Ensuring the drug reaches the target site in the body.
- High Cost: R&D for drug design is expensive and time-consuming.
Future of Drug Design
The future of drug design looks promising due to:
- Artificial Intelligence (AI) and machine learning for faster and more accurate predictions
- Personalized medicine based on a patient’s genetic makeup
- CRISPR and gene editing technologies
- Big data and cloud computing for managing complex datasets
- Green chemistry for safer and eco-friendly drug development
Conclusion
Drug design is a key pillar of modern pharmaceutical research. It helps in creating targeted, safer, and more effective medicines. With the help of various scientific approaches, such as ligand-based, structure-based, computer-aided, and fragment-based design, researchers are now able to discover and develop drugs in a faster and more systematic way.
Understanding these approaches not only highlights the complexity of drug discovery but also shows the exciting future ahead in medical science.
Frequently Asked Questions (FAQs)
Q1. What is the goal of drug design?
Ans: The goal is to create molecules that can effectively interact with biological targets to treat or prevent diseases.
Q2. What is the difference between ligand-based and structure-based drug design?
Ans: Ligand-based design uses data from known active compounds, while structure-based design relies on the 3D structure of the target protein.
Q3. Why is computer-aided drug design important?
Ans: It reduces time and cost by predicting drug-target interactions using software, making drug discovery more efficient.
Q4. Can natural products be used in drug design?
Ans: Yes, many drugs are inspired by or directly derived from natural products.
Q5. What are the key challenges in drug design?
Ans: Drug resistance, side effects, low bioavailability, and high development costs are major challenges.




Fantastic site. Lots of useful information here. I?¦m sending it to several buddies ans additionally sharing in delicious. And of course, thanks on your sweat!
Helpful overview
Thank you