Phenanthrene, a polycyclic aromatic hydrocarbon, showcases diverse chemical properties that underscore its significance in organic chemistry and industrial applications:
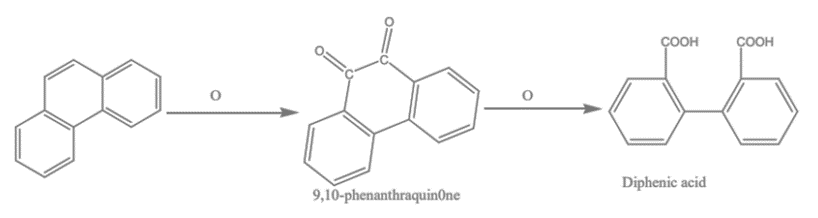
1. Oxidation of Phenanthrene: When phenanthrene is oxidized with chromic acid in acetic acid, it produces a diketone called phenanthraquinone, which can be further oxidized to form diphenic acid.
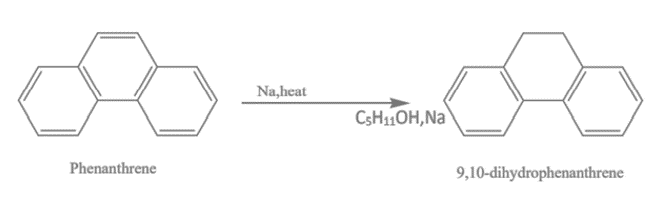
2. Reduction of Phenanthrene: When sodium and isopentanol are used to reduce Phenanthrene, 9,10-dihydrophenanthrene is produced.
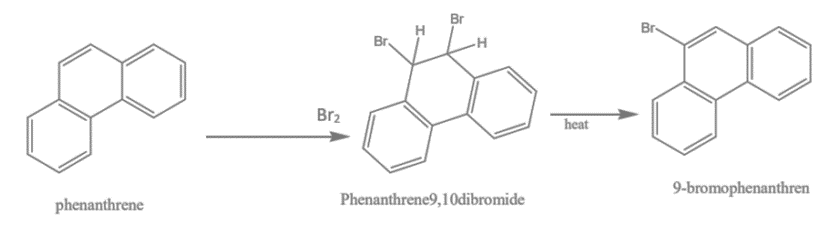
3. Bromination of phenanthrene: When phenanthrene is reacted with bromine, it produces a compound called 9-Bromophenanthrene.
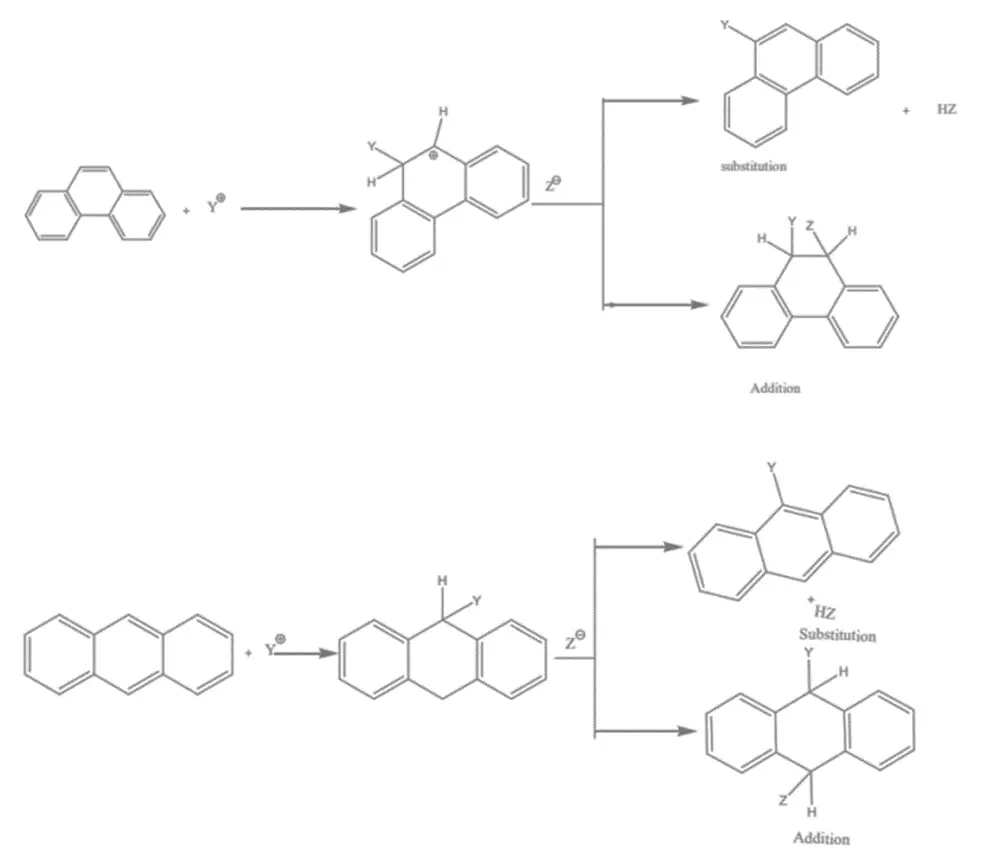
4. These compounds’ 9- and 10- positions are reactive towards electrophilic attack, which can lead to either substitution or addition. This reactivity is due to the formation of a carbocation that is the most stable one, preserving aromatic sextets in two of the three rings. This carbocation can either give up a proton to yield the substitution product or accept a nucleophile to yield the addition product. These compounds tend to undergo addition due to the comparatively small sacrifice in resonance energy, which is 12 kcal/mol for anthracene and 20 kcal/mol or less for Phenanthrene.
Conclusion
In conclusion, the chemical properties of phenanthrene reveal its versatility in various reactions. It forms phenanthraquinone and diphenic acid through oxidation, while reduction yields 9,10-dihydrophenanthrene. Bromination leads to the production of 9-Bromophenanthrene. Notably, the reactivity of the 9- and 10- positions towards electrophilic attack results in substitution or addition reactions. This reactivity is attributed to the stability of the carbocation formed, preserving aromatic sextets in two of the three rings. Phenanthrene compounds typically undergo addition reactions due to a modest sacrifice in resonance energy, emphasizing their importance in organic synthesis and industrial processes.




