Amino acids are the building blocks of life. They form proteins involved in nearly every biological process in the human body. From muscle development to enzyme function and immune responses, amino acids are at the centre of it all.
Each amino acid contains a basic structure, but different side chains give each one unique properties and roles. Out of the hundreds of amino acids, only 20 standard amino acids are used to make proteins in humans.
This post will explain amino acids in a simple, human-like manner by covering:
- What amino acids are
- Their classification
- Their chemical structure
- Their essential roles in the body
What are Amino Acids?
Amino acids are organic compounds that combine to form proteins. They are called “amino acids” because they contain both an amino group (-NH₂) and a carboxylic acid group (-COOH).
They are essential for:
- Building proteins
- Creating enzymes and hormones
- Repairing tissues
- Supporting metabolism and immunity
There are 20 main amino acids encoded by the human genetic code.
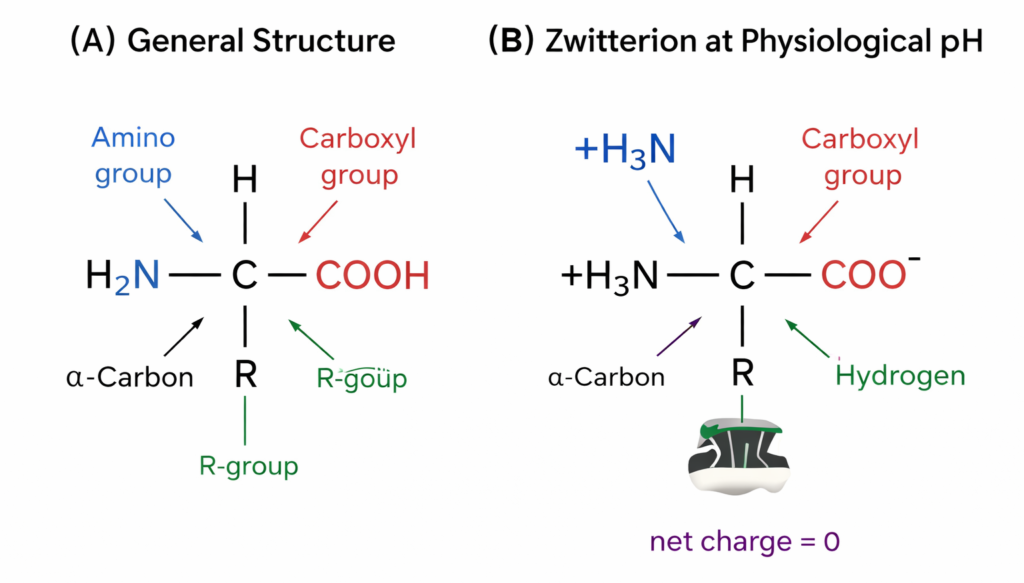
General Structure of an Amino Acid
Each amino acid has the same basic structure:
- Amino group (-NH₂)
- Carboxylic acid group (-COOH)
- Hydrogen atom (H)
- A variable R-group (side chain) attached to a central carbon (called the α-carbon)
General Formula:
H₂N – CH(R) – COOH
- The R-group (side chain) defines the unique characteristics of each amino acid.
- Glycine, for example, has a hydrogen (-H) as its side chain, while phenylalanine has a bulky benzyl group.
Classification of Amino Acids
Amino acids can be classified in multiple ways depending on their structure, function, and nutritional requirements.
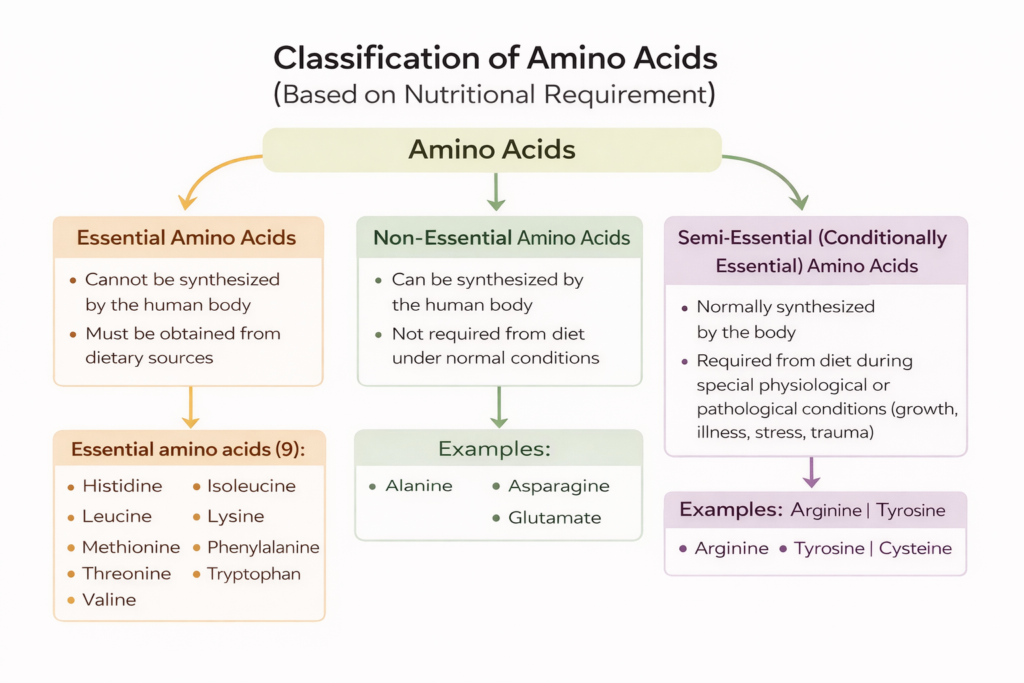
1. Based on Nutritional Requirement: This classification is based on whether the body can synthesize the amino acid or must get it from the diet.
a. Essential Amino Acids
- Cannot be made by the body
- Must be obtained from food
- There are 9 essential amino acids: Histidine, Isoleucine, Leucine, Lysine, Methionine, Phenylalanine, Threonine, Tryptophan, and Valine
b. Non-Essential Amino Acids
- Can be synthesized by the body
- Examples: Alanine, Asparagine, Glutamate
c. Semi-Essential (Conditionally Essential)
- Required in special conditions (e.g., during growth, illness)
- Examples: Arginine, Tyrosine, Cysteine
2. Based on the Nature of the R-group (Side Chain)
| Group | Examples | Features |
| Nonpolar (Hydrophobic) | Glycine, Alanine, Valine | Insoluble in water |
| Polar (Uncharged) | Serine, Threonine | Can form hydrogen bonds |
| Acidic (Negatively charged) | Aspartic acid, Glutamic acid | Contain carboxyl side chains |
| Basic (Positively charged) | Lysine, Arginine, Histidine | Contain amino side chains |
| Aromatic | Phenylalanine, Tyrosine, Tryptophan | Contain ring structures |
3. Based on Metabolic Fate
- Glucogenic Amino Acids: Convert into glucose (e.g., alanine, serine)
- Ketogenic Amino Acids: Convert into ketone bodies (e.g., leucine, lysine)
- Both: Isoleucine, phenylalanine, tyrosine
Chemical Nature of Amino Acids
1. Zwitterion Nature
At physiological pH (around 7.4), amino acids exist as zwitterions, which means:
- The amino group is protonated to -NH₃⁺
- The carboxylic group is deprotonated to -COO⁻
- The molecule carries both positive and negative charges but is overall neutral
This gives amino acids unique properties such as:
- High melting points
- Solubility in water
- Buffering capacity
2. Optical Activity
- All amino acids (except glycine) have a chiral carbon (α-carbon), making them optically active.
- They exist as L- and D-isomers
- Only L-amino acids are used in human proteins
3. Peptide Bond Formation
Amino acids link together through peptide bonds:
- The -NH₂ group of one amino acid reacts with the -COOH group of another
- A molecule of water is removed (condensation reaction)
- This forms a dipeptide, and repeated linking creates polypeptides and eventually proteins
4. Acid-Base Behavior
- Amino acids can act as buffers, resisting changes in pH
- Each has an isoelectric point (pI): the pH at which it carries no net charge
Biological Role of Amino Acids
Amino acids are involved in almost every vital function in the body. Their importance goes far beyond just forming proteins.
1. Building Blocks of Proteins
- Proteins are made up of long chains of amino acids
- The sequence of amino acids determines a protein’s structure and function
- Proteins function as: Enzymes, Hormones, Structural components, and Transport molecules
2. Enzyme Production
- Many enzymes (biological catalysts) are proteins made of amino acids
- These speed up biochemical reactions like digestion, DNA replication, etc.
3. Hormone Synthesis
- Some amino acids act as precursors for important hormones
- Tyrosine → Thyroxine (thyroid hormone)
- Tryptophan → Serotonin, Melatonin
- Arginine → Nitric oxide (vasodilator)
4. Immune Function
- Amino acids like glutamine support immune cells
- Proteins formed from amino acids serve as antibodies that fight infections
5. Muscle Repair and Growth
- Branched-chain amino acids (BCAAs: leucine, isoleucine, valine) help in muscle repair and growth
- Athletes often consume BCAA supplements for faster recovery
6. Energy Source
- In extreme situations (starvation, heavy exercise), amino acids can be converted to glucose or used as fuel
- Especially important when carbohydrate stores are low
7. Detoxification
- Amino acids are involved in removing waste products like ammonia
- Example: Glutamine helps carry excess nitrogen to the liver for detoxification
8. Neurotransmitter Synthesis
- Many neurotransmitters are derived from amino acids
- Glutamate: Excitatory neurotransmitter
- GABA (from glutamate): Inhibitory neurotransmitter
- Dopamine and serotonin: Mood regulators
9. Transport and Storage of Nutrients
- Some amino acids help in transporting nutrients across cell membranes
- They also act as precursors for other bioactive molecules
Common Dietary Sources of Amino Acids
| Food | Amino Acids Present |
| Eggs | All essential amino acids |
| Meat (chicken, beef) | Leucine, isoleucine, valine |
| Fish | Lysine, methionine |
| Dairy (milk, cheese, yogurt) | Tryptophan, threonine |
| Legumes (beans, lentils) | Histidine, lysine |
| Soy and tofu | Complete protein |
| Nuts and seeds | Arginine, glutamine |
Health Implications of Amino Acid Deficiency or Excess
Deficiency Can Cause:
- Weak immunity
- Muscle loss
- Fatigue
- Mood swings and depression
- Poor wound healing
Excess Intake May Lead To:
- Kidney strain (in people with kidney problems)
- Imbalance of amino acids
- Digestive issues
Applications in Medicine and Biotechnology
- Amino acid supplements: For bodybuilders, elderly people, and patients with metabolic disorders
- Medical nutrition: Amino acid-rich diets for post-surgery recovery
- Biotechnology: Used in making drugs, enzymes, and industrial chemicals
- Disease markers: Abnormal amino acid levels help diagnose metabolic diseases like phenylketonuria (PKU)
Conclusion
Amino acids are vital to life. They are much more than just “building blocks of proteins”—they help regulate metabolism, support immunity, control mood, and keep our bodies functioning. Understanding their classification, chemical nature, and biological roles is important in fields such as pharmacy, medicine, nutrition, and biochemistry. Eating a balanced diet with quality protein sources ensures your body gets all the essential amino acids it needs.
Suggested Reading
- “Lehninger Principles of Biochemistry”
- “Harper’s Illustrated Biochemistry”
- “Nutrition: Concepts and Controversies” by Frances Sizer
Frequently Asked Questions (FAQs)
Q1. How many amino acids are there in total?
There are 20 standard amino acids used to build proteins in the human body.
Q2. What is the difference between essential and non-essential amino acids?
- Essential: Must come from food
- Non-essential: Made by the body
Q3. What is a peptide bond?
A chemical bond is formed between the carboxyl group of one amino acid and the amino group of another.
Q4. Which amino acids help build muscle?
Leucine, isoleucine, and valine called BCAAs are crucial for muscle growth and recovery.
Q5. What are complete proteins?
Foods that contain all 9 essential amino acids, like eggs, milk, meat, and soy.