Tetracyclines have been trusted antibiotics for over 70 years, and they are known for their broad-spectrum activity against various bacterial infections. While natural tetracyclines like tetracycline and oxytetracycline are great, science has created semisynthetic tetracyclines- chemically modified versions with improved properties.
In this blog post, we’ll focus on the two most important semisynthetic tetracyclines: minocycline and doxycycline. You’ll learn how they’re made, how they work, what makes them better than natural tetracyclines, and how they’re used in real-world medicine.
What Are Semisynthetic Tetracyclines?
Semisynthetic tetracyclines are chemically modified forms of natural tetracyclines. The modifications are done to:
- Improves absorption in the body
- Increased half-life (stays longer in the system)
- Enhance effectiveness against resistant bacteria
- Reduce side effects like GI irritation
The most common semisynthetic tetracyclines are:
- Minocycline
- Doxycycline
- (Others include methacycline, rolitetracycline, etc., but we’ll focus on the two main ones.)
Chemical Structure and Molecular Properties
All tetracyclines have a four-ring backbone. But semisynthetic versions have slight modifications on the rings to improve their pharmacokinetics and stability.
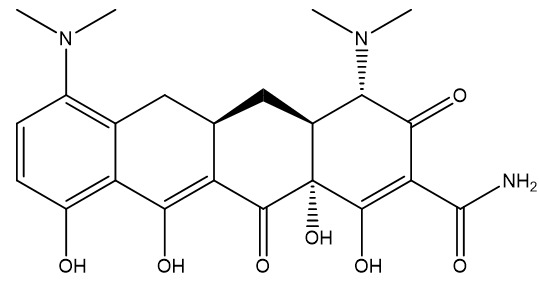
1. Minocycline
- Chemical Formula: C23H27N3O7
- Molecular Weight: 457.5 g/mol
- Key Modifications: Dimethylamino group at C-7 position
- Solubility: Slightly soluble in water, soluble in alcohol
- Chemical structure:
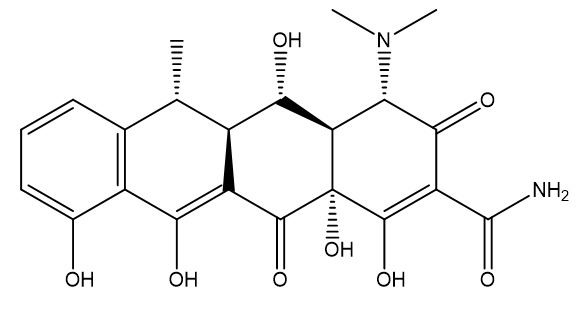
2. Doxycycline
- Chemical Formula: C22H24N2O8
- Molecular Weight: 444.4 g/mol
- Key Modifications: Hydroxyl group at C-5, lacks the hydroxyl at C-6 (makes it more stable in acidic environments)
- Solubility: Freely soluble in water
- Chemical structure:
Synthesis: How Are They Made?
Semisynthetic tetracyclines are made by chemically modifying natural tetracyclines like tetracycline or oxytetracycline. Here’s how it’s done:
Minocycline Synthesis
- Starts from tetracycline
- Undergoes chlorination and methylation reactions
- Substitutions made at the C-7 position of the D ring
- These changes increase lipophilicity, making it more absorbable and effective in tissues
Doxycycline Synthesis
- Begins with oxytetracycline
- Involves removal of the hydroxyl group at position C-6
- Addition of hydrogen to prevent degradation in acidic pH
- The final product is highly bioavailable and stable
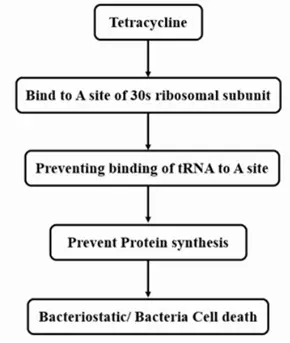
Mechanism of Action
Like all tetracyclines, minocycline and doxycycline work by inhibiting protein synthesis in bacteria. Here’s how:
- They enter bacterial cells by passive diffusion and active transport.
- Bind to the 30S subunit of bacterial ribosomes
- Prevent the attachment of tRNA to the ribosome
- This stops the bacteria from producing proteins, eventually killing or stopping their growth
Pharmacokinetics Comparison
| Feature | Minocycline | Doxycycline |
| Half-Life | ~16–18 hours | ~18–22 hours |
| Bioavailability | ~90–100% (excellent) | ~95% (very high) |
| Protein Binding | ~70–90% | ~80–90% |
| Excretion | Mostly via liver (bile) | Mostly via feces |
| Food Interaction | Less affected | Not significantly affected |
This is why both are commonly prescribed as once- or twice-daily doses—super convenient for patients.
Spectrum of Activity
Minocycline and doxycycline are broad-spectrum antibiotics, effective against:
Gram-positive bacteria:
- Staphylococcus aureus (including MRSA)
- Streptococcus pneumoniae
Gram-negative bacteria:
- E. coli
- Haemophilus influenzae
- Neisseria gonorrhoeae
Atypical pathogens:
- Chlamydia trachomatis
- Mycoplasma pneumoniae
- Rickettsia
- Borrelia (Lyme disease)
Other uses:
- Acne (long-term use)
- Malaria prophylaxis (doxycycline)
- Periodontitis (minocycline gel)
Medical Uses
1. Minocycline
- Moderate to severe acne vulgaris
- Skin and soft tissue infections
- Meningococcal carrier state
- RA (Rheumatoid Arthritis) – as a disease-modifying agent
- Periodontitis (used as a topical gel)
- Multidrug-resistant bacterial infections
2. Doxycycline
- Acne (mild to moderate)
- Respiratory infections (bronchitis, pneumonia)
- Sexually transmitted infections (Chlamydia, gonorrhea)
- Malaria prophylaxis
- Rickettsial infections (Rocky Mountain spotted fever, typhus)
- Anthrax exposure treatment
Both are also used in dental infections, urinary tract infections, and tick-borne illnesses.
Side Effects and Precautions
1. Common Side Effects
- Nausea or vomiting
- Diarrhea
- Photosensitivity (sunburn risk)
- Dizziness (more with minocycline)
- Esophageal irritation (take with water)
2. Serious Side Effects
- Hepatotoxicity (rare)
- Vestibular toxicity (minocycline)
- Superinfections (Candida, C. difficile)
- Tooth discoloration (in children)
3. Not Recommended For:
- Pregnant women
- Breastfeeding mothers
- Children under 8 years (tooth development interference)
Resistance Issues
Like other antibiotics, overuse has led to resistance:
- Bacteria use efflux pumps to push the drug out
- Some modify ribosomes so tetracyclines can’t bind
- Enzymatic inactivation
To combat this, newer glycylcyclines (like tigecycline) have been developed. But minocycline and doxycycline still remain highly effective in many resistant cases—especially MRSA.
Differences Between Minocycline and Doxycycline
| Feature | Minocycline | Doxycycline |
| Lipophilicity | Higher | Moderate |
| Tissue Penetration | Better (especially CNS) | Very good |
| Half-Life | Slightly shorter | Slightly longer |
| Dizziness Risk | Higher (vestibular) | Lower |
| Malaria Use | Not commonly used | Used in prevention |
| Topical Forms | Yes (gel for gums) | Not common |
Facts
- Minocycline has anti-inflammatory and neuroprotective effects, which are being studied for diseases like ALS and Parkinson’s.
- Doxycycline is a WHO essential medicine due to its effectiveness and affordability.
Storage and Handling
- Store at room temperature, away from direct sunlight.
- Don’t use expired tetracyclines; they may become toxic to the kidneys.
- Always complete the prescribed course to prevent resistance.
Conclusion
Minocycline and doxycycline are brilliant examples of how slight chemical changes can make a good antibiotic even better. They are more stable, easier to take, and work against a wider range of infections than natural tetracyclines.
From acne to malaria and STDs to respiratory infections, these two antibiotics are still making a difference today—and will likely continue to play a role in modern medicine for years to come.
📢 Found this helpful? Share it with classmates or friends who are studying antibiotics! Want us to cover another drug or class? Drop your request in the comments or contact us.
Frequently Asked Questions (FAQs)
1. Is doxycycline better than minocycline?
Both have their strengths. Doxycycline is better tolerated and used for malaria, while minocycline is more effective for acne and penetrates tissues better.
2. Can I take doxycycline with food?
Answer: Yes, unlike older tetracyclines, doxycycline can be taken with food to reduce stomach upset. Just avoid dairy or antacids close to the dose.
3. Why does minocycline cause dizziness?
Answer: Minocycline has higher lipophilicity and can cross into the brain more easily, which may affect the inner ear and balance system.
4. Are they safe during pregnancy?
Answer: No. Tetracyclines can harm fetal bone and tooth development, so they’re avoided in pregnancy and children under 8.
5. Can tetracyclines be used for viral infections like colds or flu?
Answer: No. They are antibiotics, which means they only work against bacteria—not viruses like the flu or common cold.




